Background: Community-acquired respiratory viruses such as influenza (Flu), human metapneumovirus (hMPV), parainfluenza virus (PIV), and respiratory syncytial virus (RSV) are leading causes of morbidity and mortality after hematopoietic cell transplant (HCT) or solid organ transplant (SOT). While these viruses may begin in the upper respiratory tract, ~50% of cases progress to the lower respiratory tract, potentially leading to more severe conditions such as pneumonia, bronchiolitis, bronchiolitis obliterans, and chronic lung allograft dysfunction.
ALVR106 is an allogeneic, off-the-shelf, polyclonal, multi-respiratory virus T-cell therapy specific for Flu, hMPV, PIV, and RSV. ALVR106 is generated by exposing peripheral blood mononuclear cells to peptide mixtures followed by ex vivo expansion in presence of activating cytokines. The expanded T cells are Th1-polarized, polyfunctional, and selectively able to kill viral target cells. ALVR106 cells have not shown alloreactivity, attesting to their selectivity and safety for use in HCT or SOT.
Methods: In this first-in-human, placebo (PBO)-controlled, dose-ranging trial, HCT or SOT recipients 17 years or older with upper or mild lower respiratory tract infections (URTI/LRTI) caused by Flu, hMPV, PIV, or RSV were randomized in a 3:1 ratio to receive ALVR106 or PBO. Four doses of ALVR106 ranging from 1 × 10 5 cells/kg to 2 × 10 6 cells/kg were planned to be given to 4 cohorts of 4 patients each. Patients receive up to 2 infusions of ALVR106 or PBO once every 14 days and will be followed for up to 1 year.
The primary objective was safety as measured by treatment emergent adverse events (AEs), AEs of special interest (acute and chronic graft vs host disease (GvHD), cytokine release syndrome (CRS), infusion related reactions (IRR), and progressive dyspnea), and clinical laboratory abnormalities from baseline (BL) through 12 weeks after the last infusion. Secondary endpoints included evaluation of antiviral activity by qualitative PCR assay by nasal swab and a composite endpoint through D28 which included change from BL in Radiation Therapy Oncology Group Lung Toxicity Scale Grade and viral detection from nasal swab. Patients with an improvement in symptoms or undetectable virus were considered partial responders (PR); those with improved symptoms and undetectable virus were complete responders (CR), and those whose condition did not improve or worsen from BL were non-responders (NR). To assess functional T-cell immune reconstitution and frequency of circulating virus-specific T cells, IFN-g virus-specific ELISpot analysis was performed on serial blood samples over time. ELISpot results will be presented. Preliminary study results presented herein will be unblinded at the time of data presentation.
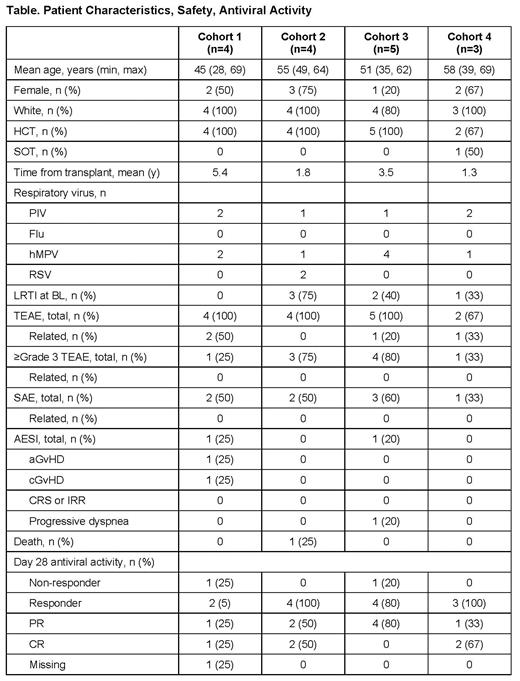
Results: 16 patients were dosed: four in Cohorts (C) 1 and 2, five in C3, and three in C4. Table shows BL demographics, disease characteristics, and blinded results for safety and antiviral activity by composite endpoint. Cohorts 2, 3, and 4 had at least 1 patient with mild LRTI at BL, no patients in C1 had LRTI. All patients received at least 1 infusion. No patients with Flu were enrolled.
In these blinded results, no differences were seen in rate or severity of AEs among cohorts. Four patients (25%) had AEs possibly related to treatment (ALVR106 or PBO); all were Grade 1 (mild) in severity. These related events were reported in 1 patient each: diarrhea, ear pressure, neutrophil count decrease, and 1 patient had pyrexia, cGvHD oral, and aGvHD skin. Eight patients (50%) had serious AEs (SAEs), none related to treatment. One patient (6%) in C3 had G2 progressive dyspnea not related to treatment. One patient (6%) in C1 had Grade I aGVHD skin and cGVHD oral; both were considered possibly related to treatment (ALVR106 or PBO). No patients had Grade II-IV aGVHD, cGVHD, IRR, or CRS. No clinically significant changes from BL or trends over time in lab abnormalities or vital signs were seen. At Day 28, all patients in C1 and C2 had undetectable respiratory virus via nasal swab, except 1 patient in C1 who missed D28 visit due to COVID-19; 4/5 (80%) in C3 were undetectable; and 2/3 (67%) in C4 were undetectable.
Conclusion: In this ongoing trial-the first-in-human, double-blind, PBO-controlled trial of virus-specific T cell therapy in transplant recipients with respiratory infection-ALVR106 was safe and well tolerated, supporting its continued evaluation in HCT and SOT recipients.
Disclosures
Nikiforow:Kite Pharma, Legend Biotech, Iovance, Smart Immune, Sobi: Honoraria. Stanton:AlloVir: Current Employment, Current holder of stock options in a privately-held company. Ma:AlloVir: Current Employment, Current holder of stock options in a privately-held company. Vasileiou:AlloVir: Consultancy. Gilmore:AlloVir: Current Employment, Current holder of stock options in a privately-held company. Dholaria:Atara: Research Funding; NCI: Research Funding; Pluri Biotech: Consultancy; AstraZeneca: Research Funding; Boxer Capital: Consultancy; Poseida: Research Funding; gamida cel: Consultancy; Pfizer: Research Funding; Wugen: Research Funding; ADC therapeutics: Consultancy, Honoraria; Angiocrine: Research Funding; Orca Bio: Research Funding; MEI: Research Funding; Adicet: Research Funding; Allovir: Research Funding; Poseida: Research Funding; Lumanity: Consultancy; Ellipsis pharma: Consultancy; BEAM therapeutics: Consultancy; Molecular Templates: Research Funding; Gilead: Research Funding; Takeda: Research Funding; Arivan: Consultancy; Janssen: Consultancy, Honoraria, Research Funding; BMS: Research Funding. Hanna:Sanofi: Speakers Bureau; SOBI: Speakers Bureau; EDITAS: Research Funding; Vertex: Honoraria. Taplitz:Karius, Merck: Membership on an entity's Board of Directors or advisory committees.